

Affiliations
ABSTRACT
Early administration of adjuvant chemotherapy for tumour patients has a positive impact on patients’ quality of survival and may improve long-term outcomes. However, chemotherapy also results in a variety of side-effects, of which cardiotoxicity is the most serious complication. We report a case of cardiotoxicity after postoperative chemotherapy for primary cardiac angiosarcoma. In the process of tumour treatment, attention should not be paid to the effect of tumour treatment only but also to the damage of chemotherapy drugs on the heart. In addition, this case also demonstrates the extensive use of multiparametric cardiac MRI in pre-treatment and post-treatment follow-up of cardiac tumours.
Key Words: Angiosarcoma, Chemotherapy, Cardiotoxicity, Cardiac magnetic resonance.
INTRODUCTION
Early administration of adjuvant chemotherapy for tumour patients has a positive impact on patients’ quality of survival and may improve long-term outcomes.1 However, chemo-therapy also results in a variety of side-effects, of which cardiotoxicity is the most serious complication. We report a case of cardiotoxicity after postoperative chemotherapy for primary cardiac angiosarcoma. The study protocol was approved by the Ethics Committee of Chengdu Fifth People's Hospital (AF/54/2020-02.3), and the patient signed informed consent before each examination.
CASE REPORT
A 41-year female patient with cardiac angiosarcoma presented with primary symptoms of panic, cardiac fatigue, and tightness of breath for more than 10 days. Cardiac magnetic resonance (CMR) showed a rounded mass in the right atrial cavity near the posterior wall with clear borders, measuring about 38 × 36 × 42 mm in size, which showed a broad basal sign with the posterior wall of the right atrium (Figure 1). Although she had clinical cardiovascular symptoms, cardiac function and left ventricular strain parameters were within normal limits at this time.
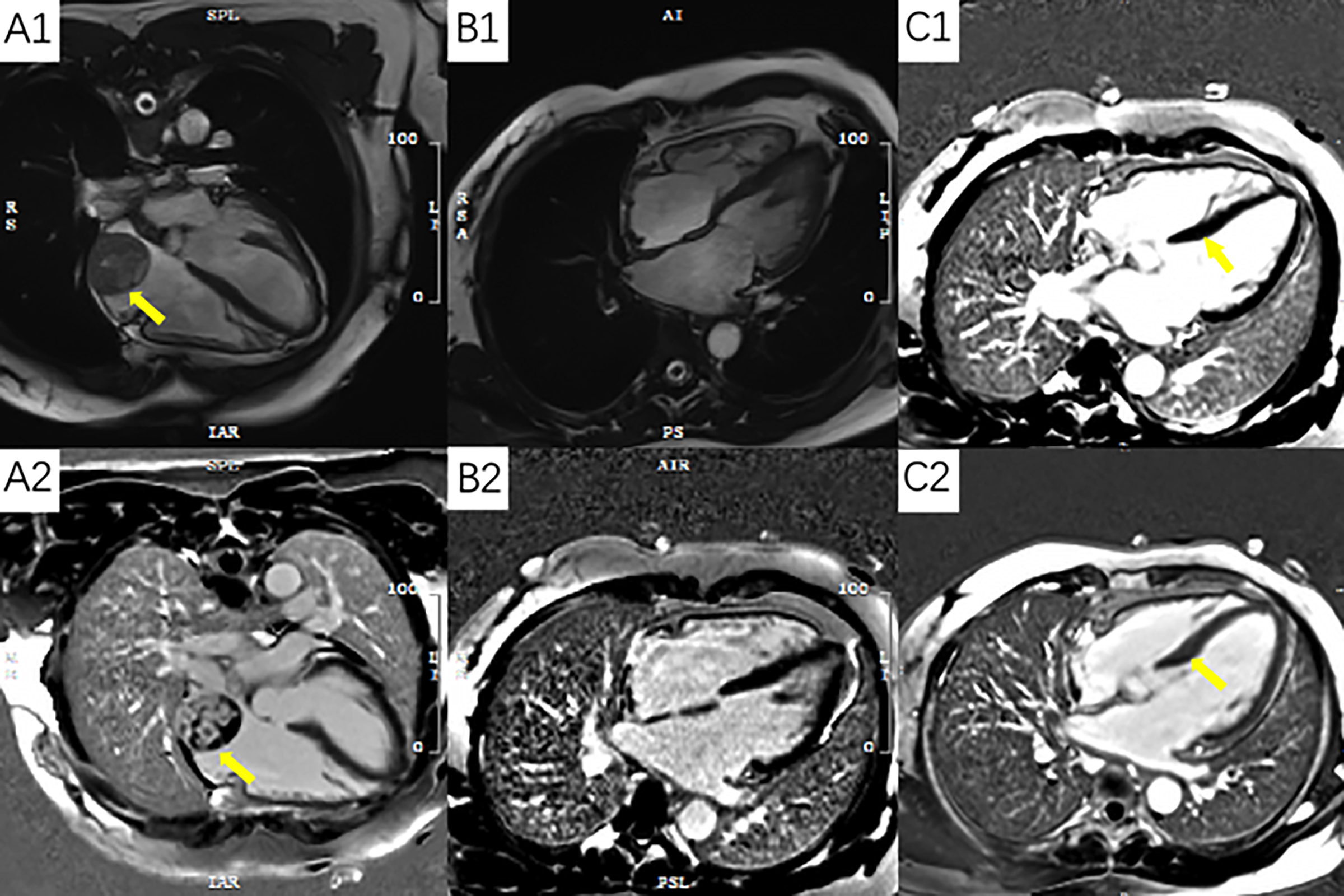 Figure 1: Panel A1 and A2 show angiosarcoma of the right atrium; Panel B1 and B2 show postoperative changes; Panel C1 is a preoperative image with no delayed enhancement of the ventricular septum; Panel C2 is a post-chemotherapy image that shows the delayed intensification of the septum.
Figure 1: Panel A1 and A2 show angiosarcoma of the right atrium; Panel B1 and B2 show postoperative changes; Panel C1 is a preoperative image with no delayed enhancement of the ventricular septum; Panel C2 is a post-chemotherapy image that shows the delayed intensification of the septum.
The patient underwent thoracoscopic extracorporeal circulation cardiac tumour resection, recovered well and was discharged home after the surgery.
After the surgery, the patient's symptoms were significantly relieved. Paclitaxel monotherapy was administered according to the regimen postoperatively, administered as 250 mg to 400 mg intravenously. Also, the patient underwent CMR (3.0-T unit, Magnetom Vida, Siemens, Erlangen, Germany) scan and enhancement (Gadobeglumine, 0.2 mmol/kg, 2.0 ml/s) every 3 months for the first year after the surgery, and six monthly thereafter. Although no tumour recurrence was seen, the patient's heart function was significantly impaired as chemotherapy progressed. After 7 sessions of chemotherapy, 18 months after the surgery, the patient developed palpitations.
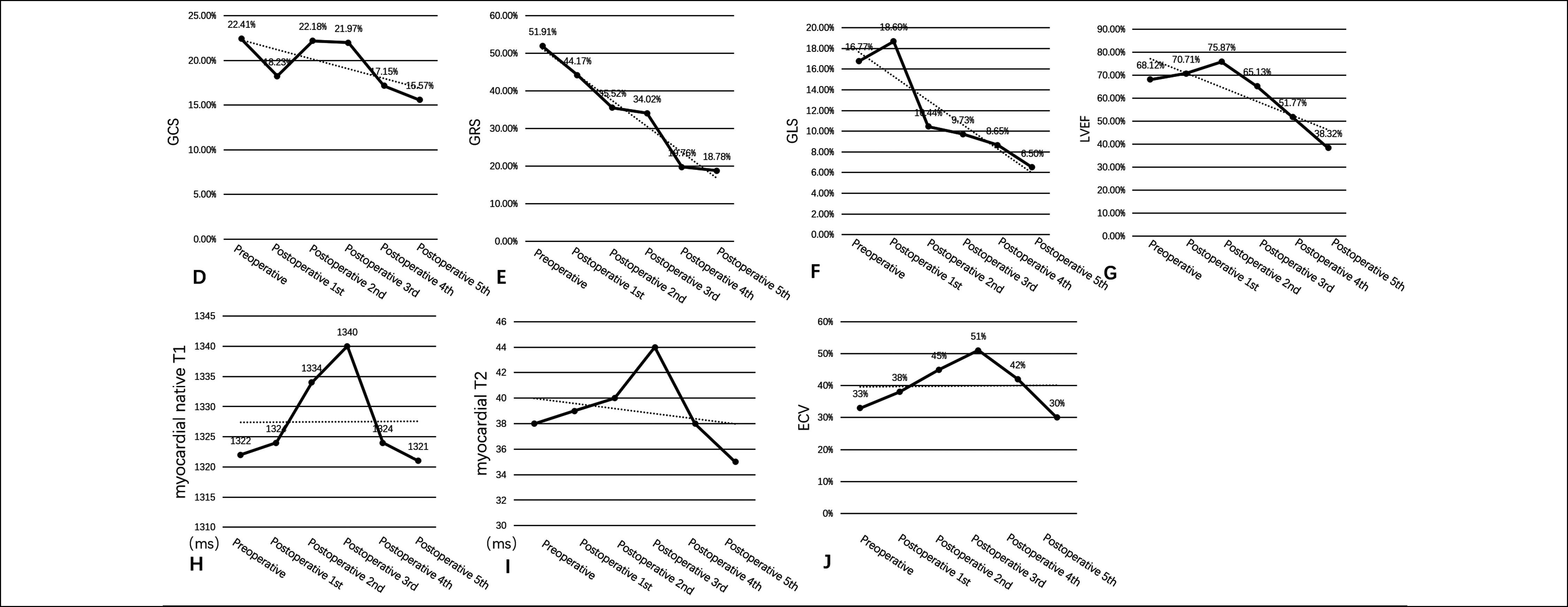 Figure 2: Panel A-D show changes in myocardial strains (GCS, GRS and GLS) and left ventricular ejection fraction (LVEF) decrease with the number of chemotherapy sessions; Panel E-G show changes in native T1, T2, and extracellular volume (ECV) values.
Figure 2: Panel A-D show changes in myocardial strains (GCS, GRS and GLS) and left ventricular ejection fraction (LVEF) decrease with the number of chemotherapy sessions; Panel E-G show changes in native T1, T2, and extracellular volume (ECV) values.
Further findings indicated that changes in the left ventricular myocardial strains occurred earlier than the decrease in the left ventricular ejection fraction (LVEF). In the early stage, T1 mapping, T2 mapping, and extracellular volume (ECV) also detected myocardial oedema (Figure 2). Based on the patient's medical history, treatment, cardiac function and cardiac parameters, the oncology and cardiovascular teams conducted a comprehensive assessment of the patient and used symptomatic treatment for the patient's cardiovascular symptoms. The patient's symptoms were relieved after relevant treatment. During the follow-up, the team of experts plan to carry out a complete reassessment of the patient's cardiovascular parameters and, develop a new treatment plan on the basis of the results.
DISCUSSION
Cancer is the second leading cause of death worldwide. Early detection and treatment can significantly improve the survival rate of patients.2 Chemotherapy can improve outcomes and cure cancer patients, but it also leads to a variety of side-effects, of which, cardiotoxicity is the most serious complication.3 Patients present with asymptomatic to severe cardiac insufficiency and irreversible heart failure, ultimately leading to high morbidity and mortality.3
Cardiac angiosarcoma is the most common cardiac malignant tumour with an extremely poor prognosis, which can occur in any chamber of the heart, but is more common in the right atrium. Early administration of adjuvant chemotherapy has a positive impact on the quality of patient survival, and chemotherapy is effective for metastases from angiosarcoma, improving long-term outcomes.1,4 Although paclitaxel-like drugs are effective in the treatment of angiosarcoma, there is no unified clinical standard, and experiments on paclitaxel damage to the heart are rare. In the present case, we can see that both LVEF and myocardial strains [including global circumferential strain (GCS), global radial strain (GRS), and global longitudinal strain (GLS)] became lower as the number of chemotherapy sessions increased (Figure 2). Native T1, T2, and ECV values peaked at 1 year after chemotherapy supporting the hypothesis of injury-related myocardial oedema (Figure 2).5
Echocardiography is the most commonly used test to assess cardiac injury, where LVEF can be used as a criterion for judging cardiac damage. However, subclinical cardiac injury occurs before LVEF changes.6 CMR is a non-invasive, comprehensive assessment of cardiac anatomy, tissue characteristics and cardiac function, and is highly accurate and reproducible, making it the gold standard for cardiac function measurement parameters.7 CMR enables a comprehensive assessment of the cardiac function and injury. Myocardial strains enable earlier and more sensitive detection of cardiotoxic injury; a reduction in GLS greater than 15% is considered an early indicator of myocardial toxic damage.8 Also, CMR tissue characterisation imaging not only monitors for tumour recurrence but also assesses cardiac function, allowing for early identification and detection of cancer therapy-related cardiovascular injury. Muehlberg et al. showed that T1 mapping, T2 mapping, and ECV can detect myocardial tissue damage earlier than the conventional functional indices.9 Galán-Arriola et al. suggested that myocardial oedema is the earliest marker of anthracycline-induced cardiotoxicity and can be detected in the T2 mapping sequence.10 All of the above can be quantitatively analysed by CMR.
In the process of tumour treatment, we should not only pay attention to the effect of tumour treatment but also to the damage of chemotherapy drugs on the heart. In addition, the present case also demonstrated the usefulness of multiparametric CMR in pre-treatment and post-treatment follow-up of cardiac tumours.
PATIENT’S CONSENT:
Informed consent was obtained from the patient.
COMPETING INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
MW: Substantial contribution to the conception and design of the work.
XM: Revising the work critically for important intellectual content.
YL: Analysis and interpretation of data for the work.
ZY, BF: Agreement to be accountable for all aspects of the work.
All authors approved the final version of the manuscript to be published.
REFERENCES